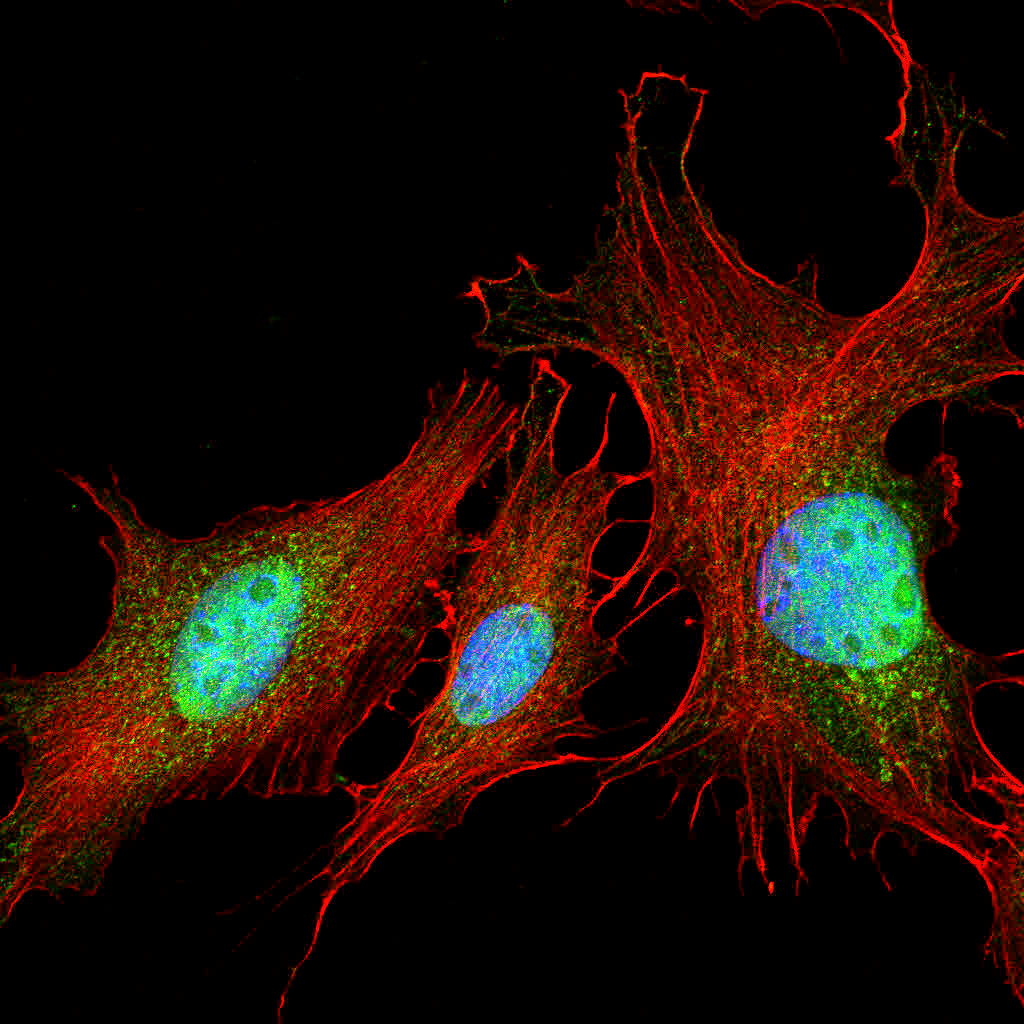
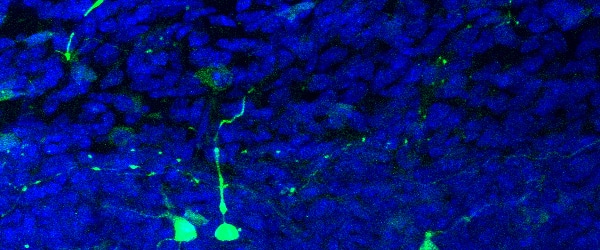
Frozen Section Immunofluorescence Protocol

Fix for 5 minutes in acetone 20 c.
Frozen section immunofluorescence protocol. Tissue should initially float and is fully impregnated with sucrose when it sinks. Otransfer tissue to 30 sucrose in a conical tube or screw cap vial and store at 4 c overnight. Use of forceps may damage tissue integrity.
This protocol is also suitable for 40µm free floating. Protocol for immunofluorescent staining of mouse frozen sections tissue. Mount tissue sections onto gelatin or poly l lysine coated slides by placing the cold sections onto warm slides.
Paraffin and frozen sections reagents can be applied manually by pipette or this protocol can be adapted for automated and semi automated systems if these are available. Allow the temperature of the tissue to equilibrate with the cryostat. Immediately snap freeze fresh tissue in isopentane mixed with dry ice and keep at 70 c.
Preparing frozen sections. Do not allow frozen tissue to thaw before cutting. Cut cryostat sections at 5 10 µm and mount on gelatin coated histological slides.
Section the frozen tissue block into a desired thickness typically 5 10 µm using the cryotome. Tissue transfers should be performed delicately with a slotted spoon or reagent scoop. Cryosections adhered to slides from blocks embedded in oct using the 2 methylbutane isobutene method.
Slides can be safely stored for 6 12 months at 80 c until ready for staining. Carry out incubations in a humidified chamber to avoid tissue drying out which will lead to non specific binding and high background staining. Sections can be stored in a sealed slide box at 80 c for later use.