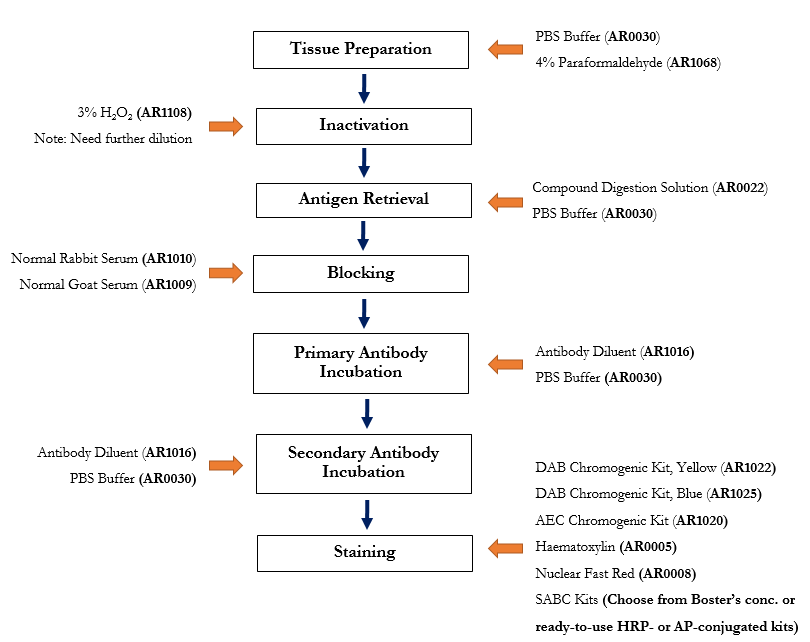
Tissue Sectioning Protocol

Ucsbmicroscopy lifesci ucsb edu to set up a training time with mary raven fo r cryostat use.
Tissue sectioning protocol. Certain soft tissues such as brain are optimally frozen in m 1 medium at 3 c. Tissue sections may be dried by onto microscope slides and stored for extended periods at room temperature. Floor of bio ii in the microscopy facility.
1 fischer ah jacobson ka rose j zeller r. Paraffin sectioning and mounting protocol glen macdonald january 6 2000 the result of this procedure for mounting sections is to allow any section from a sample to be equated with its position in the sample tissue or relative to other sections in the series. Machine is located on the 5.
The speed in which the frozen sectioning is accomplished is improved on several levels. If necessary adjust the temperature of the cutting chamber 5 c according to the tissue under study. The tissue can be difficult to section if the tissues have variable textures water or fatty.
After the training you will be allowed to sign up online to use the machine. Frozen tissues are prepared by immersing the tissue in liquid nitrogen isopentane or by burying the sample in dry ice. Tissue sections are then rehydrated prior to commencing the immunostaining protocol.
Allow the slides to dry overnight and store slides at room temperature until ready for use. The system provides distinct advantages over available methods for embedding tissue for frozen section including speed precision reduced tissue wastage ease of learning and convenience. Sectioning of paraffin embedded tissue video protocol embedding tissue into paraffin blocks supports the tissue structure and enables very thin sections to be cut and mounted onto microscope slides for analysis.
Sample with tissue identity. Do not allow frozen tissue to thaw before cutting. Section paraffin blocks at the desired thickness usually 4 5 µm on a microtome and float on a 40 c water bath containing distilled water.