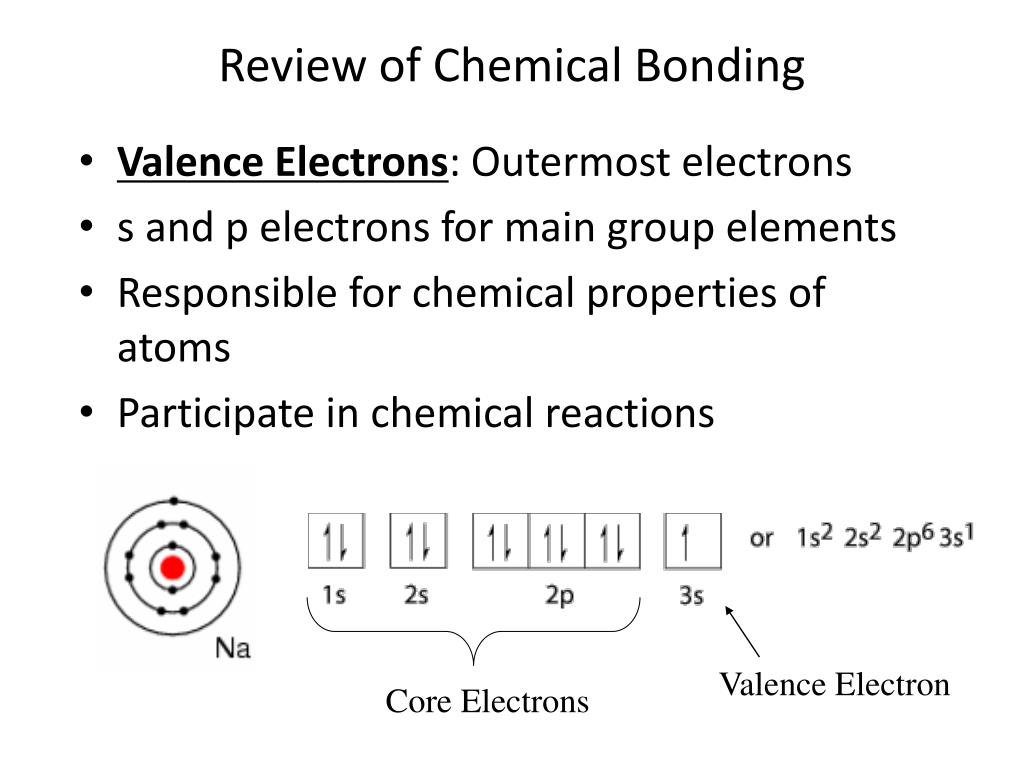
6 1 Introduction To Chemical Bonding Section Review Answers

Chemical bonding is one of the most basic fundamentals of chemistry that explains other concepts such as molecules and reactions.
6 1 introduction to chemical bonding section review answers. In chapter 6 we will begin studying how atoms interact with each other to form chemical bonds. Single bond sigma bond double bond. A a chemical bond between atoms results from the attraction between the valence electrons and of different atoms.
Learn vocabulary terms and more with flashcards games and other study tools. B a covalent bond consists of a a shared electron. Explain why most chemical bonding is neither purely ionic or purley 5.
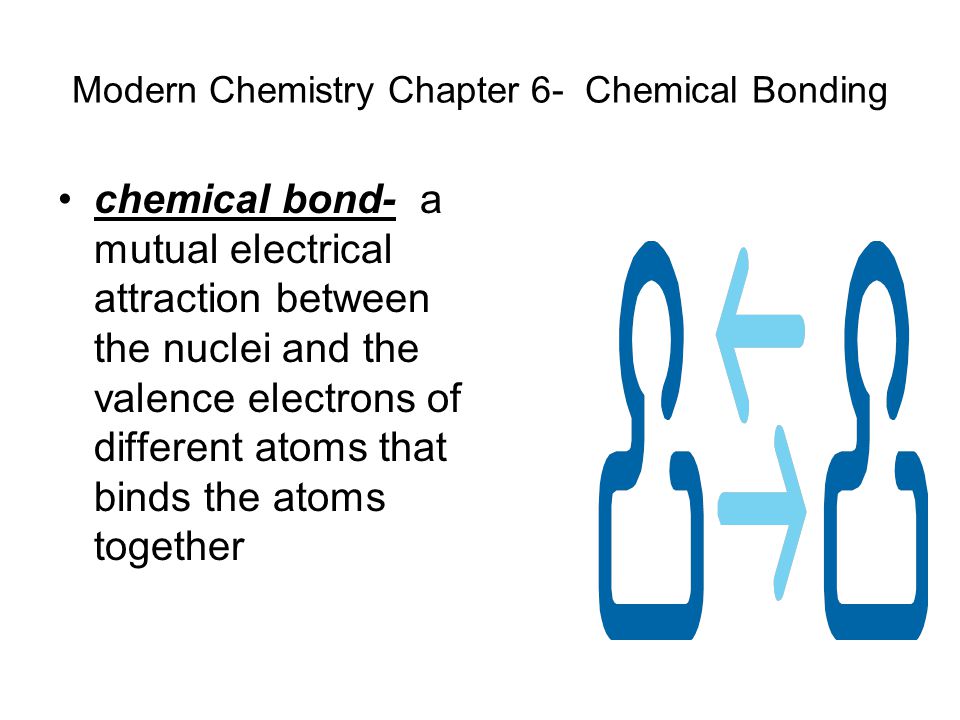
Types of chemical bonding ionic or covalent. Chemical bonds a mutual electrical attraction between the nuclei and valence electrons of different atoms that binds the atoms together. Classify bonding type according to electronegativity differences.
Chemical bond that results from the electrical attraction between cations and anions ions. For example a boulder is less likely to balance at the top of a hill than it is to roll. The valency of an element is a the combining capacity of one atom of it b the number of page 2 5.
Section 6 1 introduction to chemical bonding answer key 1. When they do they form chemical bonds. Because when they bond with each other atoms are more stable and have a lower potential energy.
Chemical bonding test review answer key part 1 answers. The way the electrons are redistributed determines the type of bond. A nuclei c isotopes b inner electrons d lewis structures 2.